Editorial
This month: epistemology, motor control and automated patch clamp.
First, why epistemology in a theoretical neuroscience journal? Because epistemology of neuroscience is theoretical neuroscience. It is about reflecting on what it means to model behavior or the nervous system, what methods and metaphors (eg “coding”) are relevant conceptual tools. It sets the frame in which meaningful questions can be asked and theories can be built. What is it that we want to explain when we make a model? Do we want to explain experimental data? If at a cocktail party I am asked about my work, I might respond for example that I try to understand how we localize sounds in space. Crucially, I do not respond that I develop models that try to explain the percentage of errors in a given psychophysical task when hearing tones through headphones in the lab. Yet in most studies, and in particular theoretical studies, we tend to forget the big picture. The model matches some experimental data, but does it actually address the hard problem, i.e., to explain real behavior? In the field of sound localization, most models are hopelessly bad at anything remotely related to sound localization in real settings, but they are good at discriminating tones (Goodman et al., 2013). We simply forget that a model of a sensory system is meant to explain how animals do the awesome things that they do, and not only to match a set of lab data on a trivial task (discriminating tones). Matching artificial experimental data provides contraints on models, but it is not the goal. Krakauer et al. (2) make this point in a recent essay and argue for more thorough studies of behavior (I would even say, ethology). An older paper by Tytell et al. (3) goes further and argues that one needs to realize that the nervous system is embodied and interacts with the physical world, and behavior is the result of this interaction. Crucially, it appears that the nervous system can tune the body, not only control it.
This last point has motivated us to look at how muscles produce movement and force. This is the subject of a theoretical paper by my student Charlotte Le Mouel where we argue that posture is actually tuned not for equilibrium but for potential movement (1). Muscles are controlled by spikes, and this control is often given as an example of rate coding. This in my view is an example of the confusions between correlation and causation often seen in the spike vs. rate debate (see my essay on the subject, Brette 2015). A nice 2006 study by Zhurov and Brezina (5) demonstrated in Aplysia that actually, spike timing is crucial in determining both the temporal pattern and the amplitude of muscular contraction, which is a deterministic function of spike pattern. A recent paper shows that it also appears to be the case in vertebrates (4).
Finally, this issue features 4 papers on automated patch-clamp (6-9). All have been published in the last 5 years. Why is this relevant to a theoretical neuroscience journal? Because I believe this might allow theoretical neuroscientists to dig into experiments themselves, which would be extremely beneficial. Patch-clamp is tedious, technical and labor-intensive. It is difficult to do both serious theory (and by this, I mean not only simulating models but also analyzing them and making predictions) and patch clamp experiments to test it. But for a few years now, it has become possible to automate most of the process – one must still prepare the tissue, the solutions, and pull electrodes. What is missing currently is: open source software for the automation, and perhaps a reduction of hardware costs (currently very expensive) using open hardware (eg 3D printed parts).
From the lab
1. Le Mouel C and Brette R (2017). Mobility as the purpose of postural control. (Comment on PubPeer).
As a first step into the development of sensorimotor models (for example orientation responses), we have looked at how muscles produce movement and force. This paper explains which muscles you should contract and in which order so as to produce certain movements efficiently, using elementary mechanical considerations (ie, we do not need muscle physiology). We then show how it explains muscular contraction patterns that are observed experimentally in humans in a variety of situations. Quite surprisingly (at least for us), we have found that posture seems to be adjusted not for stability per se, but to allow for efficient movements to be performed when necessary (eg when balance is perturbed). The work also questions the theory of muscular synergies, as it shows that skillful movement requires fine muscular control, both spatially and temporally.
Articles
2. Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D (2017). Neuroscience Needs Behavior: Correcting a Reductionist Bias. (Comment on PubPeer).
From the perspective of a computational neuroscientist, I believe a very important point is made here. Models are judged on their ability to account for experimental data, so the critical question is what counts as relevant data? Data currently used to constrain models in systems neuroscience are most often neural responses to stereotypical stimuli, and results from behavioral experiments with well-controlled but unecological tasks, for example conditioned responses to variations in one dimension of a stimulus. This leads to models that might agree with laboratory data (by design) but that don’t work, i.e. that do not explain how the animal manages to do what it does. I have made this point in the specific context of sound localization (Brette, 2010; Goodman et al., 2013). More on PubPeer and Pubmed Commons.
3. Tytell ED, Holmes P, Cohen AH (2011). Spikes alone do not behavior make: why neuroscience needs biomechanics. (Comment on PubPeer).
This review makes the point that behavior results not only from neural activity but also from the mechanical properties of the body, or more broadly from the coupling between body and environment. A famous example in robotics is McGeer’s passive walker. The paper draws on many interesting examples from (mostly but not only) insects locomotion. I found that the most interesting part of this review was the discussion of active tuning of passive properties. That is, one way in which animals produce movement is not by directly controlling the different limbs, as we would imagine if we were to control a robot, but by modulating the passive mechanical properties of the musculoskeletal system. For example, if two antagonists muscles are contracted, they become stiffer, which changes their reactions to perturbations. These reactions are instantaneous, as they do not require the nervous system; these are called “preflexes”. The paper ends on the idea that the development of motor skill might rely on the tuning of preflexes, rather than on the development of central control. This opens very interesting paths for theoretical neuroscience.
4. Srivastava KH, Holmes CM, Vellema M, Pack A, Elemans CPH, Nemenman I, Sober SJ (2017). Motor control by precisely timed spike patterns. (Comment on PubPeer).
This study shows that the precise spike timing of vertebrate motoneurons has significant behavioral effect, by looking at breathing in songbirds, which is slow compared to the time scale of spike patterns. Long recordings are obtained with an MEA, together with air pressure and force recordings. Focusing on 20-ms bursts of 3 spikes, they show that shifting the middle spike by a few milliseconds has strong effects on muscle contraction and air pressure, due to nonlinearities in the neuromuscular transform. The findings support the view that firing rates correlate with various aspects of neural activity, but spikes causally determine neural activity and behavior (Brette 2015). This is a nice study, although the authors seem to have missed a previous study that shows very similar findings with more detail in an invertebrate (Aplysia) (Zhurov and Brezina, 2006).
5. Zhurov Y and Brezina V (2006). Variability of Motor Neuron Spike Timing Maintains and Shapes Contractions of the Accessory Radula Closer Muscleof Aplysia. (Comment on PubPeer).
This study shows that the precise spike timing of motoneurons controlling a feeding muscle of Aplysia has strong effect on its contraction. This is surprising because that muscle is a slow muscle that contracts over seconds, but adding or removing just one spike has a very strong and immediate effect on contraction, as shown in this figure (Fig. 1C):
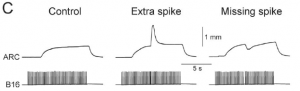
The muscle is controlled by just two neurons, so it is a nice model system. The authors also show that natural spike patterns are irregular, but the neuromuscular transform is deterministic, which means that shifting spikes has a reproducible effect on the pattern of contraction, which is not just a temporal shift but also a strong change in amplitude, due to nonlinear effects. The result is that natural patterns produce twice more contraction than regular patterns of the same rate. In addition, these irregular patterns appear to be synchronized across the two sides of the animal, producing synchronized contractions. This is very convincing and supportive of spike-based theories of neural function (Brette 2015).
6. Kodandaramaiah SB, Franzesi GT, Chow BY, Boyden ES, Forest CR (2012). Automated whole-cell patch-clamp electrophysiology of neurons in vivo. (Comment on PubPeer).
This is the first demonstration of automatic patch-clamp in intact cells (i.e., not with patch clamp chips which work with suspensions). It was done in vivo, which is actually simpler than in vitro because it is blind: the pipette is lowered until a cell is detected, which is signaled by an increase in resistance. The full code and circuit designs are freely available, although the code is in Labview, proprietary software; it is also made for specific hardware (amplifier and acquisition board), although this can of course be adapted. An update with more detail has been recently published (Kodandaramaiah et al. 2016). The key element is the pressure controller, which allows the program to send positive or negative pressure and suction pulses through the pipette. There is a clever design in this study, which is very cheap to build: there are 4 tanks with specified pressures (I suppose using large pipettes that are manually filled with air), and a few electrovalves controlled by an acquisition board switch between the different tanks.
7. Desai NS, Siegel JJ, Taylor W, Chitwood RA, Johnston D (2015). MATLAB-based automated patch-clamp system for awake behaving mice. (Comment on PubPeer).
This is similar to the blind in vivo automatic patch-clamp technique of Kodandaramaiah et al. (2016), with a few differences. One is that it is written in Matlab, also proprietary software. The more interesting difference, in my view, is the pressure controller. Instead of using 4 manually filled tanks, there is an automatic electronic system that adjusts the pressure to any specified value. It essentially mixes two pressure sources (+10 psi and -10 psi) using a PID controller programmed on an Arduino. The code is also freely available.
8. Wu Q, Kolb I, Callahan BM, Su Z, Stoy W, Kodandaramaiah SB, Neve R, Zeng H, Boyden ES, Forest CR, Chubykin AA (2016). Integration of autopatching with automated pipette and cell detection in vitro. (Comment on PubPeer).
This study adapts the automated patch-clamp technique introduced in Kodandaramaiah et al. (2016) to slices. The approach is visually guided (using simple computer vision algorithms); the motorized manipulator is also automatically calibrated with the camera, using a pipette detection algorithm. The paper claims a 2/3 success rate, instead of 1/3 for a human operator. The code is available in Labview and Python, which is nice, but unfortunately the Python code is not in any usable form at this moment (no documentation and very few comments). I regret that a lot of technical detail is missing from the paper, in particular details of the computer vision algorithms and of the pressure control system. This control system is different from the previous one; instead of tanks with fixed pressure, it seems to use a single pump and a pressure sensor in a clever way to produce both positive and negative pressure. The drawing on Fig. 2C is the only information I could find about the system in the paper.
9. Kolb I, Stoy WA, Rousseau EB, Moody OA, Jenkins A, Forest CR (2016). Cleaning patch-clamp pipettes for immediate reuse. (Comment on PubPeer).
This is a simple but very interesting study where the authors show that it is possible to clean patch clamp pipettes in Alconox up to 10 times, and reuse the pipettes on different cells with no noticeable effect. This is what was missing to truly automate patch clamp, as it was previously necessary to manually change the pipette after every recorded cell (or failed attempt).
